Technology
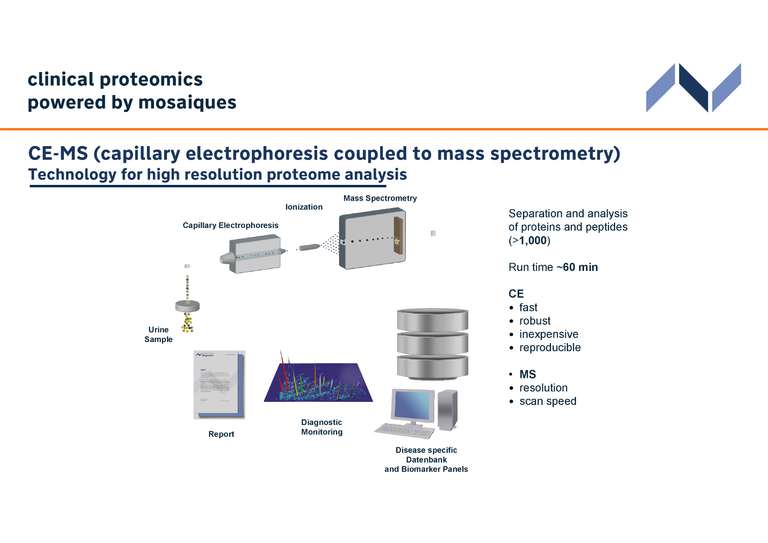
CE-MS
We are pioneers in clinical proteomics, having led the development of capillary electrophoresis coupled to mass spectrometry (CE-MS). Key breakthroughs include the creation of a stable and proprietary CE-MS technology, ensuring reproducible and comparable analyses. We have also established robust protocols for sample collection and preparation, as well as internal standards for precise data normalization. In addition, we have developed proprietary software tools to manage big data effectively, resulting in the creation of an extensive urinary proteome database. Today, our technology is employed for clinical diagnostics and biomarker identification, driving advancements that have an impact on healthcare and the future of personalized medicine.
Our CE-MS technology stands out due to its:
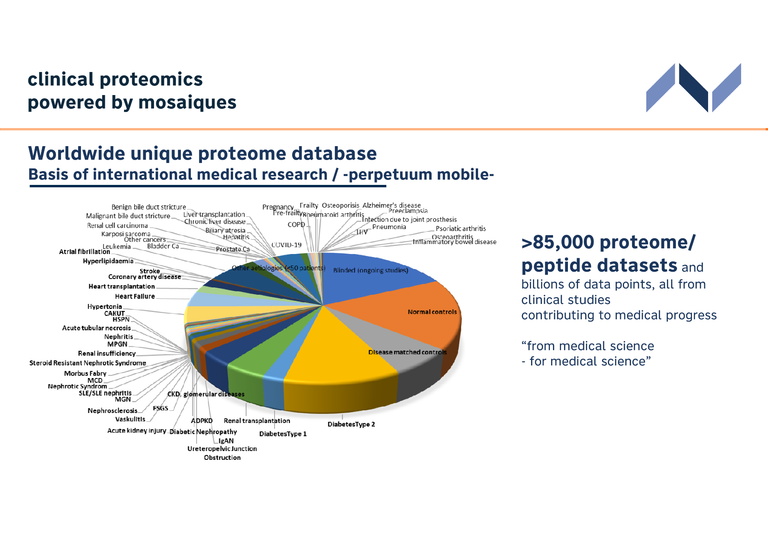
- Database with more than 85 000 datasets
- Fast, reliable, and reproducible results with high resolution and speed.
- Capability for qualitative and quantitative analysis of urinary peptides/proteins.
Worldwide unique proteome database
We are proud to present a ground-breaking achievement in the world of proteomics - a urinary proteome database that promises to revolutionize research and innovation.
The urinary proteome database holds information from >85000 urine samples and associated data. This repository offers researchers, scientists, and healthcare professionals a resource to accelerate discoveries and advance our understanding of the molecular mechanisms associated with diseases, and to develop new biomarkers. Using the power of the database, the identification of biomarkers can be performed in a targeted, fast, and efficient manner.
Further reading
Mavrogeorgis, E., Mischak, H., Latosinska, A., Siwy, J., Jankowski, V., & Jankowski, J. (2021). Reproducibility Evaluation of Urinary Peptide Detection Using CE-MS. Molecules (Basel, Switzerland), 26(23), 7260. doi.org/10.3390/molecules26237260
Latosinska, A., Siwy, J., Mischak, H., & Frantzi, M. (2019). Peptidomics and proteomics based on CE-MS as a robust tool in clinical application: The past, the present, and the future. Electrophoresis, 40(18-19), 2294–2308. doi.org/10.1002/elps.201900091
Mischak, H., Vlahou, A., & Ioannidis, J. P. (2013). Technical aspects and inter-laboratory variability in native peptide profiling: the CE-MS experience. Clinical biochemistry, 46(6), 432–443. doi.org/10.1016/j.clinbiochem.2012.09.025
Latosinska, A., Siwy, J., Mischak, H., & Frantzi, M. (2019). Peptidomics and proteomics based on CE-MS as a robust tool in clinical application: The past, the present, and the future. Electrophoresis, 40(18-19), 2294–2308. doi.org/10.1002/elps.201900091
Stalmach, A., Albalat, A., Mullen, W., & Mischak, H. (2013). Recent advances in capillary electrophoresis coupled to mass spectrometry for clinical proteomic applications. Electrophoresis, 34(11), 1452–1464. doi.org/10.1002/elps.201200708
Siwy, J., Mullen, W., Golovko, I., Franke, J., & Zürbig, P. (2011). Human urinary peptide database for multiple disease biomarker discovery. Proteomics. Clinical applications, 5(5-6), 367–374. doi.org/10.1002/prca.201000155
Wir benötigen Ihre Zustimmung zum Laden der Übersetzungen
Wir nutzen einen Drittanbieter-Service, um den Inhalt der Website zu übersetzen, der möglicherweise Daten über Ihre Aktivitäten sammelt. Bitte überprüfen Sie die Details in der Datenschutzerklärung und akzeptieren Sie den Dienst, um die Übersetzungen zu sehen.